Contributions of a combined experimental/modeling approach
Novembre 12, 2024
Although nano-alloys are of great scientific and practical interest, their formation remains poorly understood. In this study, we explore the gold-silver system by combining experiments and simulations to elucidate formation mechanisms at the atomic scale. We synthesized nanoparticles by gas-phase aggregation and performed machine-learning-assisted simulations to further our understanding of the crystallization process. Our results reveal gold segregation on the surface of nanoparticles, which we discovered to be caused by charge transfer and electrostatic interactions, challenging previous ideas.
Although nanoalloys are of great scientific and practical interest, the processes leading to their formation are not yet well understood. Key characteristics of the alloys, such as crystal phase, chemical arrangement, and morphology, are challenging to control at the nanoscale, complicating their industrial application. In this study, we focus on the gold-silver system, two of the most common noble metals, and combine experiments and simulations to uncover how these alloys form at the atomic level.
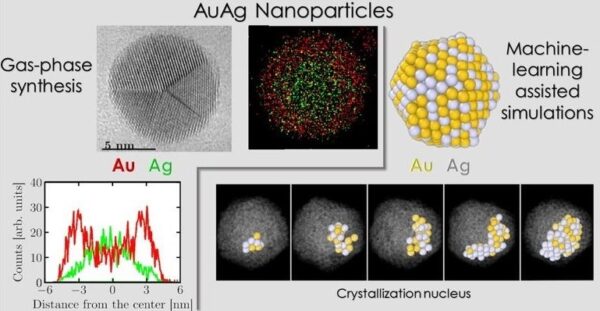
We synthesized nanoparticles using an advanced gas-phase aggregation technique and analyzed their morphology and chemical distribution at the atomic scale using transmission electron microscopy. We employed machine-learning-assisted molecular dynamics simulations to model the crystallization process, transitioning from liquid droplets to nanocrystals.
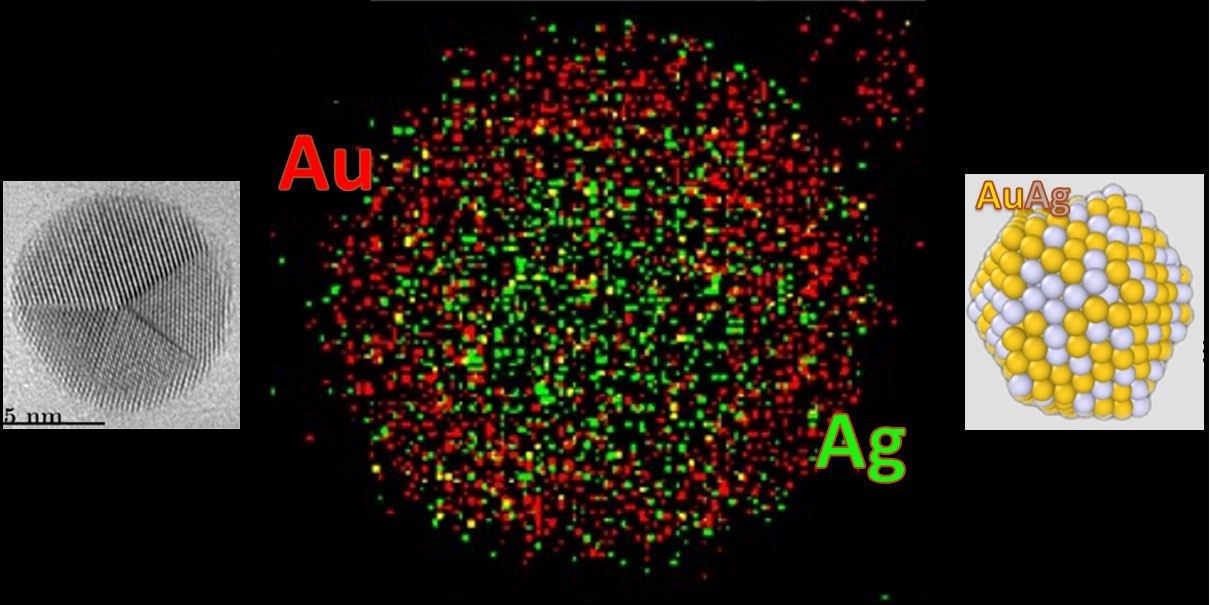
Our study reveals that, as for the monometallic nanoparticles, the majority of the formed nanoparticles exhibit a unique five-fold symmetric shape, such as icosahedra and decahedra. However, we found that gold atoms, rather than silver atoms, concentrate at the surface of the nanoparticles, regardless of the alloy composition. This segregation tendency contrasts with previous studies and influences both the crystallization dynamics and the resulting crystal arrangement. This observation challenges previous notions and could alter our understanding of the formation mechanisms of these nanoparticles.
Finally, we demonstrated that this surprising segregation dynamics is driven by charge transfer and electrostatic interactions, rather than surface energy considerations as previously thought. The use of machine learning was essential for modeling the crystallization process, allowing us to analyze the obtained data more effectively.
Graphical abstract
Contacts:
Patrick Benzo | patrizio.benzo[at]cemes.fr
Magali Benoit | magali.benoit[at]cemes.fr
Publication:
Exploring the formation of gold/silver nanoalloys with gas-phase synthesis and machine-learning assisted simulations
Quentin Gromoff, Patrizio Benzo, Wissam A. Saidi, Christopher M. Andolina, Marie-José Casanove, Teresa Hungria, Sophie Barre, Magali Benoit, and Julien Lam
Nanoscale, 2024,16, 384-393
DOI: https://doi.org/10.1039/D3NR04471H