Toward a photoinduced modulation of the oxidation state of molecules on surfaces
December 8, 2025
With an original multidisciplinary approach combining in-solution studies and on-surface investigations based principally on Scanning Probe Microscopy, the photoinduced deoxygenation of sulfoxides, a well-known process in solution, was successfully transposed to alkali halide insulating substrates. This work opens the way for photocontrolled charge state manipulation in organic molecules on surfaces.
On-surface synthesis has emerged as a powerful way to produce new functional nanomaterials with finely-tuned properties, which cannot be prepared by conventional in-solution synthetic chemistry. To date, the vast majority of on-surface reactions has been achieved on metallic surfaces, which strongly modify the optical and electronic properties of adsorbed species. Insulating substrates thus appear as highly promising alternatives for on-surface synthesis, allowing for direct investigations of intrinsic optoelectronic properties of the newly-formed nanostructures, as a starting point for their optimization and future applications.
In this context, a consortium including researchers from CEMES, LHFA (Toulouse) and IM2NP (Marseille) successfully transposed the photoinduced deoxygenation of sulfoxides, a well-known process in solution, to an alkali halide insulating surface under Ultra High Vacuum at low temperature. A precursor derived from dibenzothiophene S-oxide (DBTO), displaying optimized molecular properties, was synthesized and characterized in solution. Deposition on an ionic substrate (NaCl thin-film on Au(111)) led to the formation of organized self-assemblies, revealing the adsorption configuration of the pyramidal sulfoxide chemical group on such surfaces.
The photoreactivity of DBTO molecules was then investigated on surface using Scanning Tunneling Microscopy (STM), non-contact Atomic Force Microscopy (nc-AFM) and bias spectroscopy measurements. The combined studies showed that irradiation with UV light triggers in situ the S=O bond cleavage at the single molecule scale, thus leading chemoselectively to the same dibenzothiophene (DBT) product as obtained in solution.
During this reaction, the oxidation state of the sulfur atom within the molecule varies from (0) to (-II). The photodeoxygenation thus represents a powerful and reagentless synthetic tool allowing the formal reduction of the sulfur atom in situ. This work opens the way for the photocontrolled charge state manipulation in purely organic compounds on surfaces.
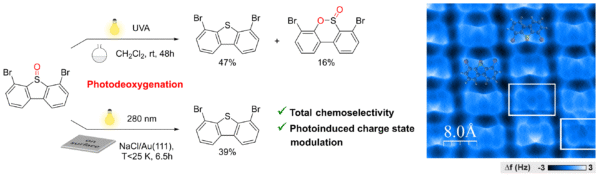
Photoinduced deoxygenation of dibenzothiophene S-oxide (o-Br)2-DBTO, transposed from solutions to alkali halide insulating surfaces, yielding chemoselectively the reduced product (o-Br)2-DBT. Right: non-contact AFM image (Ultra High Vacuum, 10 K) of a molecular monolayer composed of still unreacted (o-Br)2-DBTO precursors (some structures are superimposed) and of deoxygenated (o-Br)2-DBT products (in white frames), adsorbed on two NaCl monolayers on Au(111), after 30 min UV irradiation (280 nm).
Contact:
Claire Kammerer | claire.kammerer[at]cemes.fr
Publication:
Photoinduced Modulation of the Oxidation State of Dibenzothiophene S-Oxide Molecules on an Insulating Substrate
Hankache, V. Magné, E. Geagea, P. Simón Marqués, S. Clair, L. Giovanelli, C. Loppacher, S. Mallet-Ladeira, E. Maerten, C. Kammerer, D. Madec, and L. Nony
Nat. Commun. 16 (2025) 4841
DOI: https://doi.org/10.1038/s41467-025-60075-y

