Experimental study of nucleation and growth mechanisms
September 11, 2025
We synthesized silver nanoparticles (AgNPs) in the gas phase and investigated the effect of small amounts of reactive gases on their formation. Atomic-resolution transmission electron microscopy and in situ optical spectroscopy analyses show that introducing a low fraction of O₂ into the plasma (below 0.5%) significantly increases the surface density of AgNPs, through an ion-induced nucleation process. These results provide new insights into the role of reactive gases in nanoparticle nucleation in the gas phase.
Silver nanoparticles (AgNPs) have attracted great interest owing to their remarkable optical and antimicrobial properties. Silver has been known since antiquity for its antibacterial action, mainly related to the bactericidal effect of Ag⁺ ions, which act through complex mechanisms within living cells. Silver nanoparticles are particularly effective antimicrobial agents thanks to their high surface-to-volume ratio, which enhances their interactions with the surrounding environment. As a result, they are capable of continuously releasing significant amounts of ions over long periods.
Several methods exist for producing AgNPs — chemical routes, involving ligands, or physical approaches, yielding extremely pure particles — yet the simultaneous control of both their size and density remains a major challenge. Gas-phase synthesis offers an attractive alternative, allowing independent and precise control over these parameters. However, optimizing this method requires a thorough understanding of the underlying nucleation and growth mechanisms.
At CEMES, we investigated the influence of reactive gases, particularly nitrogen (N₂) and oxygen (O₂), on the formation of silver nanoparticles in an ultra-high vacuum gas-phase aggregation source. Our results show that nitrogen mainly acts as a carrier gas, with little or no effect on AgNP nucleation. In contrast, introducing trace amounts of oxygen into the aggregation chamber (<0.5%) strongly enhances the nucleation rate.
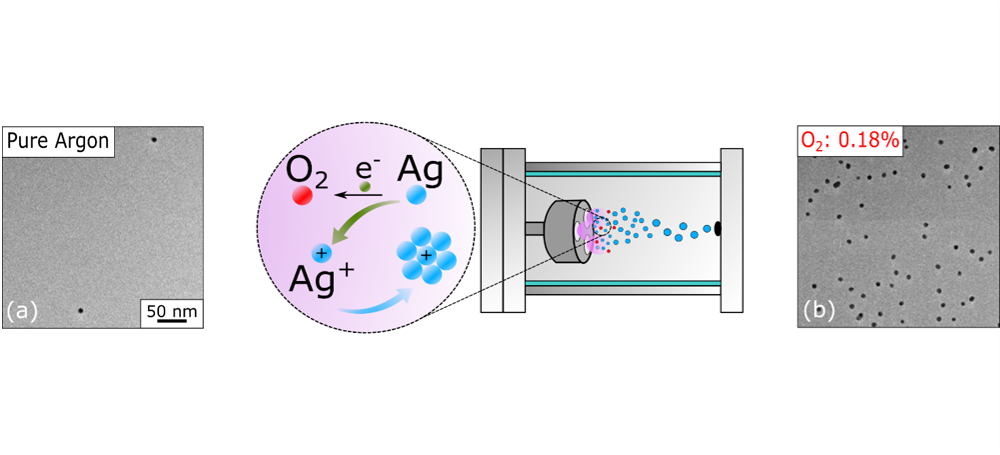
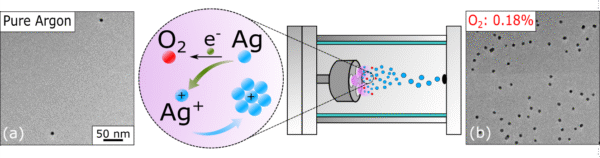 TEM images of AgNPs synthesized in gas-phase under different atmospheres: (left) using only argon without any additional gas; (right) with an oxygen concentration of 0.18%. In the center, schematic illustration of the nucleation mechanism in the presence of oxygen.
TEM images of AgNPs synthesized in gas-phase under different atmospheres: (left) using only argon without any additional gas; (right) with an oxygen concentration of 0.18%. In the center, schematic illustration of the nucleation mechanism in the presence of oxygen.
Structural analyses by high-resolution transmission electron microscopy (HRTEM) and scanning transmission electron microscopy coupled with energy-dispersive X-ray spectroscopy (STEM-EDX) confirmed that, despite the presence of O₂ during deposition, the nanoparticles retain their metallic character, with no sign of oxidation. In situ optical emission spectroscopy measurements revealed a clear increase in the concentration of excited silver species, signatures of Ag+ ions in the plasma. These observations demonstrate that oxygen plays a key role by promoting an ion-induced nucleation mechanism.
Altogether, these results provide essential insights into the dynamics of gas-phase nucleation and highlight the importance of reactive species in controlling the synthesis of metallic nanoparticles.
Contacts:
Salomé Trillot | salome.trillot[at]cemes.fr
Patrizio Benzo | patrizio.benzo[at]cemes.fr
Caroline Bonafos | caroline.bonafos[at]cemes.fr
Publication:
Role of nitrogen and oxygen in the nucleation and growth of silver nanoparticles in gas-phase synthesis
S. Trillot, P. Benzo, S. Barre, N. Tarrat, M. Benoit, K. Makasheva, and C. Bonafos
Nanoscale 17 (2025) 16796
DOI: https://doi.org/10.1039/D5NR01526J