Trapped in glass: how defects slow down silver ions
November 17, 2025
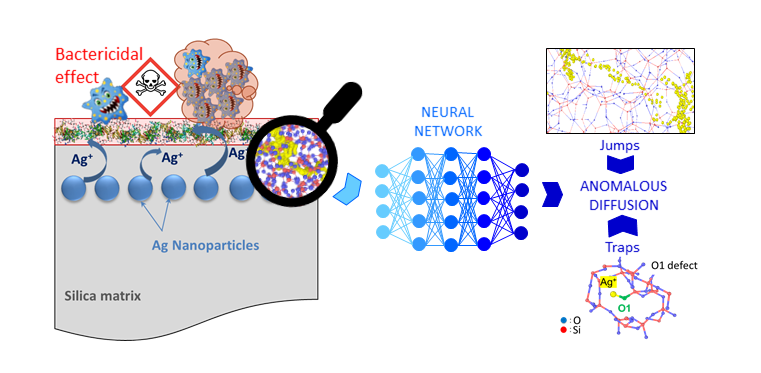
How do silver ions pass through a layer of amorphous silica? Machine learning-assisted molecular dynamics simulations show that they move in irregular jumps, sometimes trapped by defects such as undercoordinated oxygen atoms. This atomic mapping of their path paves the way for antibacte-rial coatings whose ion release can be finely controlled, thereby optimising effectiveness and dura-bility.
Surface coatings based on silica-coated silver nanoparticles have particularly promising antimicrobial properties. But how do the silver ions (Ag⁺), which are responsible for this effect, move through the thin layer of silica that covers them? A team from CEMEShas answered this question using nu-merical simulation and artificial intelligence.
The researchers performed molecular dynamics simulations based on neural-network interatomic potentials. This trained potential makes it possible to track, at the atomic scale, the movements of a silver ion within an amorphous silica matrix – a disordered material consisting of tetrahedra con-nected by their vertices.
The result: the diffusion of silver is not ‘normal’. Far from moving in a regular manner, the ion pro-gresses in irregular jumps, sometimes trapped for a long time before moving again. The origin of this behaviour, known as subdiffusion, can be traced back primariy to the disordered nature of the silica matrix and has been attributed to deceleration and temporal anticorrelations. Furthermore, this subdiffusion is amplified by the presence of coordination defects within the silica matrix. These defects, particularly under-coordinated oxygen atoms, act as traps for Ag+, forming O-Ag bonds, thereby limiting the jump length and retaining the ion for a long period of time. Compared to exist-ing diffusion models, the diffusion mechanism in the absence of defects appears to be of the Frac-tional Brownian Motion type. By comparing different silica samples, the researchers showed that the more defects the silica has, the slower the diffusion of Ag+. Furthermore, the diffusion paths during the jumps correspond to areas of lower local atomic density.
These results open up concrete prospects. By adjusting the density or number of defects in the silica layer, it would be possible to control the rate of silver ion release, and thus the effectiveness and duration of action of antibacterial coatings. This is a promising advance for the design of smart and sustainable materials.
This work is supported by the ANR BENDIS “Interaction of biological targets with solid dielectric layers consisting of silver nanoparticles embedded in silica matrices: Towards tailored antimicrobial surfaces”.
Contacts:
Magali Benoit | magali.benoit[at]cemes.fr
Nathalie Tarrat | nathalie.tarrat[at]cemes.fr
Publication:
Evidence and origin of anomalous diffusion of Ag+ ion in amorphous silica: A molecular dynamics study with neural network interatomic potentials,
S. Trillot, N. Tarrat, N. Combe, P. Benzo, C. Bonafos, and M. Benoit
J. Chem. Phys. 162 (2025) 104701
DOI: https://doi.org/10.1063/5.0251120