Towards heteromolecular covalent multilayers
November 29, 2023
2D surface synthesis makes it possible to couple molecules on monocrystalline surfaces to form new molecules or oligomers that are often unattainable by chemistry. This approach was limited to two-dimensional systems in direct contact with the substrate. It was attractive to explore the third dimension to create molecular multilayer systems with complete control over the organization of intermolecular bonds.
In this experiment, the first molecular layer is formed of 3,5-bis(carboxylic acid)-phenyl-3-maleimide (BCPM). This molecule has both a phenyldicarboxylic function designed to promote its self-assembly on the substrate, in this case an Au gold surface(111), and a maleimide ring whose activated double bond can be involved in cycloaddition reactions with one or two other carbon-carbon double bonds. The second molecule used here is a fullerene C60, as it owns a large number of potentially reactive double bonds over its entire surface.
Figure 1. Schematic diagram of 3D synthesis-on-surface
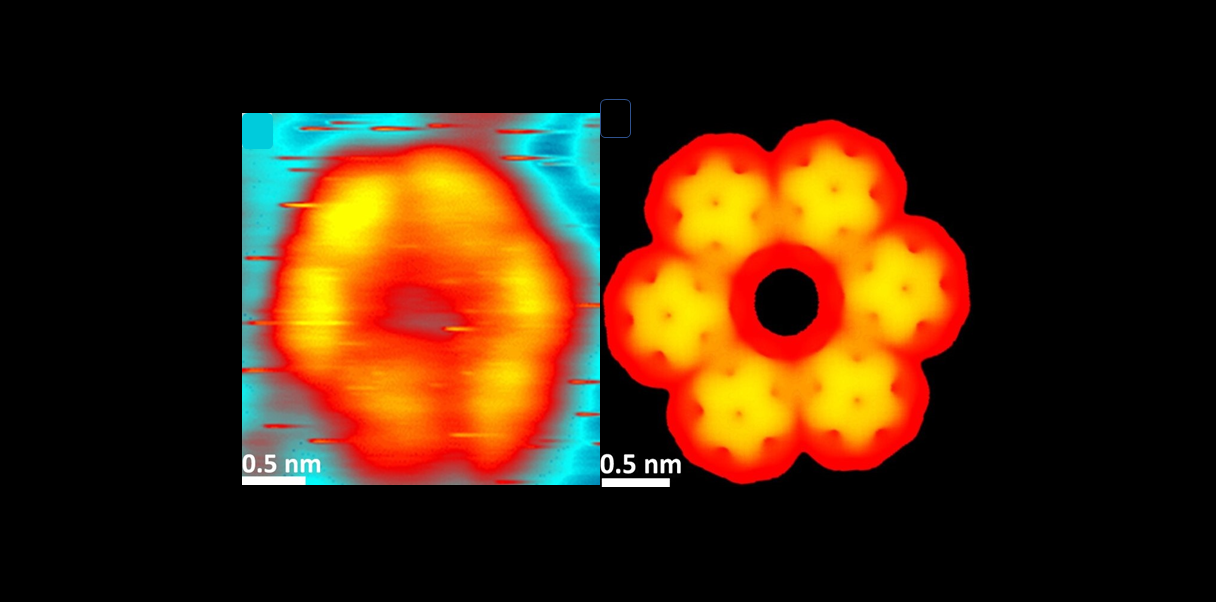
The synthesis takes place on a Au(111) gold surface in an ultra-high vacuum chamber and the evolution of the reaction is monitored by scanning tunneling microscopy (STM). The first step is the sublimation of BCPM, which self-organizes into 6-petaled flowers with a central cavity (Fig 2a). The fullerene C60 is then sublimated on this monolayer, first filling the cavities (white circles on Fig2a), then forming a monolayer with a physisorbed C60 above each BCPM (Fig2b, model in Fig2c). The free-rotating C60s appear as spheres. The temperature is then raised to 370K for 30 min, which induces a [4+2] cycloaddition reaction between the BCPM and the C60 onto which it is adsorbed. The free rotation of the C60 is then fixed and the fullerene appears as triangles in STM images (Fig 2d). Then a new annealing at 490K for 30 min allows the formation of 2 [2+2] bonds between each C60 and its two neighbors.
Here we were able to form a bi-layer heteromolecular system with complete control of covalent bonds in all three dimensions. Further extension to multilayers will give access to a wide variety of new molecular materials and devices. Fig 2. The experimental stages of 3D synthesis. Scanning tunneling microscopy images under ultra-high vacuum at 300K: a) BCPM monolayer on Au(111) after partial deposition of C60. b) and c) Full layer of C60 on BCPM/Au(111). Only the C60s above a BCPM appear in plain text here. d) Annealing at 370K/30 min induces the formation of [4+2] bonds between a C60 and each BCPM below. (e) and (f) After furher annealing at 490K/30, each C60 is covalently bonded to two of its neighbours.
Fig 2. The experimental stages of 3D synthesis. Scanning tunneling microscopy images under ultra-high vacuum at 300K: a) BCPM monolayer on Au(111) after partial deposition of C60. b) and c) Full layer of C60 on BCPM/Au(111). Only the C60s above a BCPM appear in plain text here. d) Annealing at 370K/30 min induces the formation of [4+2] bonds between a C60 and each BCPM below. (e) and (f) After furher annealing at 490K/30, each C60 is covalently bonded to two of its neighbours.
Publication:
Extending on-surface synthesis from 2D to 3D by cycloaddition with C60
P. Ding, S. Wang, C. Mattioli, Z. Li, G. Shi, Y. Sun, A. Gourdon , L. Kantorovich, F. Besenbacher, F. Rosei, and M. Yu
Nat. Commun. 14 (2023) 6075
https://rdcu.be/dojGd
Contact:
André Gourdon | andre.gourdon[at]cemes.fr