How do you create molecular "origami"?
Self-assembling structures of artificial proteins
November 13, 2023
Four French teams are demonstrating the possibility of constructing ordered supramolecular architectures that form spontaneously from proteins specially designed for this aim. The key to this innovation is the engineering of highly regular proteins with repeated motifs and recognition surfaces that enable them to establish specific interactions.
While it is possible to build nanostructures of controlled shape by exploiting the double helix structure of DNA molecules (leading to assemblies commonly known as “DNA origami”), it remains much more difficult to build such precise structures from proteins. However, in living cells, highly sophisticated supramolecular architectures such as microtubules, actin filaments and flagella perform vital functions and are entirely made up of natural proteins. They assemble spontaneously because each protein has a particular shape that allows it to interact in a very specific way with other proteins, leading to complex architectures. Creating ordered supramolecular architectures of proteins would open the door to numerous applications in biology as well as in materials science.
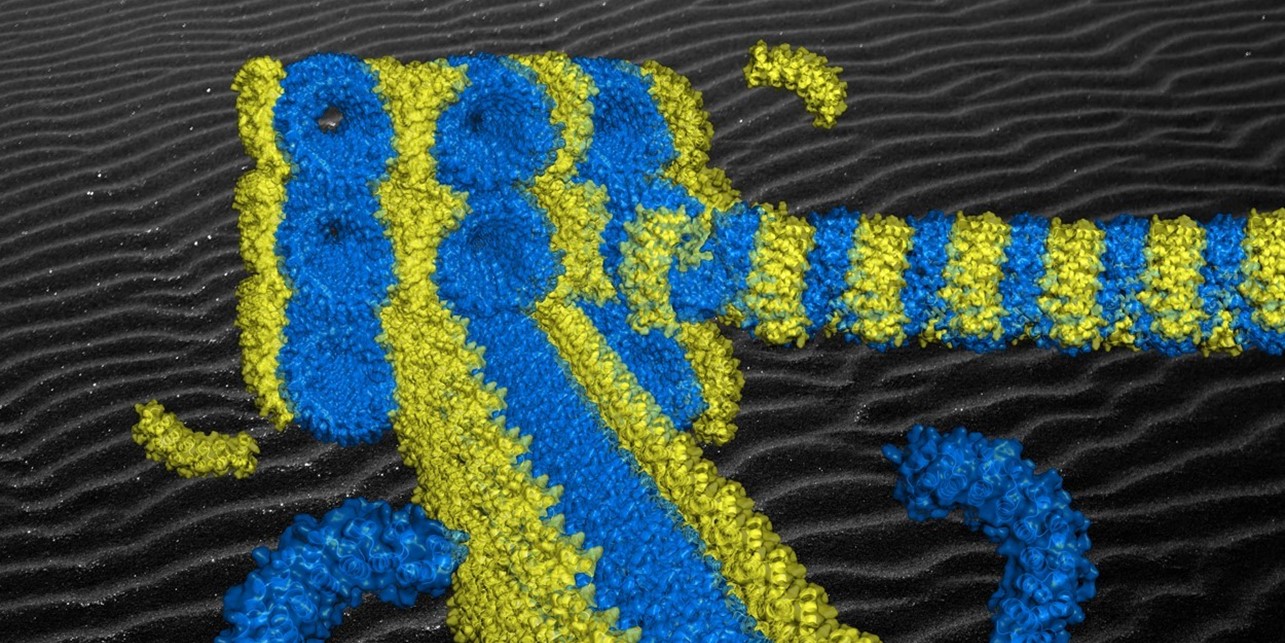
In an interdisciplinary project involving biochemists and physical chemists from CEMES (CNRS), I2BC (CNRS / CEA / Université Paris Saclay), IPR (CNRS / Université de Rennes), CBI (CNRS / Université de Toulouse – Paul Sabatier) and ICB (CNRS / COMUE Université Bourgogne Franche-Comté), a generalisable method for constructing artificial protein architectures has been developed. Rather than modifying natural proteins, the scientists have chosen to design new proteins that are both highly regular and programmed to assemble in precise geometries capable of forming stable superstructures. One of these proteins, called a ‘staple’, has the role of precisely assembling several other proteins called ‘bricks’, their assembly naturally giving to the supramolecular three-dimensional architecture its structural complexity. The first challenge was to design and produce these proteins. Then, using a combination of techniques including X-ray scattering and cryo-electron microscopy, the researchers have shown that the proteins assemble in a few minutes, at room temperature and according to the planned architecture. This is an experimental first, the principle of which can be applied to other molecular systems.
This concept of protein origami promises architecture programming that is as efficient as DNA origami, but with the added potential for widespread use that comes with the extraordinary diversity of proteins in terms of chemical functions and molecular recognition. In particular, varying the spatial arrangement of the bricks and staples could make it possible to create a whole range of ‘patterns’ for organising nano-objects with respect to to each other (enzymes, nanoparticles, viruses, etc.), encapsulating active ingredients, guiding the growth of nanomaterials or structuring the interface between biological matter and a solid material. This initial experimental work therefore opens up major prospects, especially at a favourable time when computer tools for designing artificial proteins are becoming powerful and available.
Figure – Semi-experimental model of the origami of artificial proteins in which the superhelix of bricks (in blue) self-assembles by affinity with the staple (in yellow). The extreme regularity of the supramolecular structure obtained arranges the staples periodically along the superhelix and induces the formation of aligned origami crystals @ I2BC, CEMES, CBI, IPR and ICB
Publication:
Design, synthesis, and characterization of protein origami based on self-assembly of a brick and staple artificial protein pair
L. Moreaud, S. Viollet, A. Urvoas, M. Valerio-Lepiniec, A. Mesneau, I. Li de la Sierra-Gallay , J. Miller, M. Ouldali, C. Marcelot, S. Balor, V. Soldan, C. Meriadec, F. Artzner , E. Dujardin, and P. Minard
PNAS Vol. 120 (2023) No. 11 e2218428120
https://doi.org/10.1073/pnas.2218428120
Contacts:
Cécile Marcelot | cecile.marcelot[at]cemes.fr
Erik Dujardin | erik.dujardin[at]cnrs.fr
-
Previous Post
Alain Couret
Related Posts
Wolfgang Bacsa
Enseignant chercheur UPS (PR)
Exhibition in the Boule of CEMES
June 7-22 Organised by CEMES with the support of the CNRS and the collaboration of…
Exhibition in the Boule of CEMES
June 7-22 2024





